CeGaT’s germline report is now even clearer and summarizes the most important results on the first page. The new tabular presentation brings the variant classification clearly to the point.
Clear and focused, the most significant innovations:
Page 1 – The most important information first
On the first page, you will find the result of the genetic analysis, the treatment recommendation, and the genetic relevance of the detected variant(s). This provides you with the most important information regarding your patient at a glance.
Page 2 -Variant classification with ACMG scale
The detected variant(s) are described from a molecular genetic and clinical perspective. In particular, the disease(s) associated with the gene alteration will be addressed. In addition, the ACMG criteria1 used for the variant(s) are clearly presented in a table. The variant classification is visualized using an ACMG classification scale.
Variant classification in detail
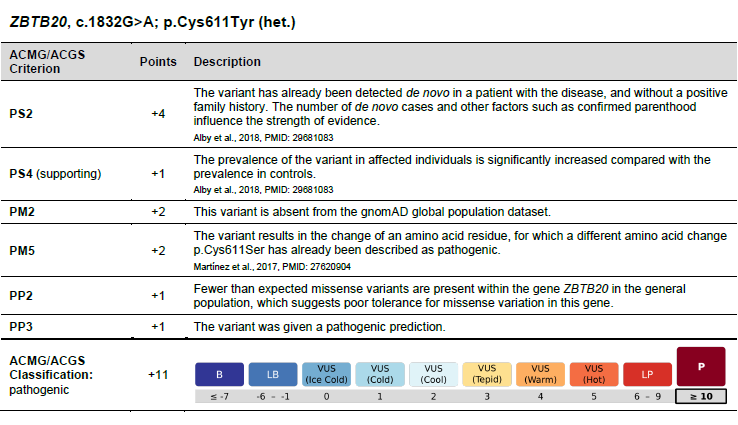
The table (see picture 3) shows the ACMG criteria used, including the number of points awarded, a detailed description and, if applicable, the specification of supporting scientific publications. This provides all relevant information on the respective variant in tabular form.
The variant classification is now visualized with a colored scale. It allows subcategorization of variants of unclear significance (VUS) according to potential disease relevance from “ice cold” to “hot”.2,3 The scale is graded from dark blue (benign; “B” for benign) to dark red (pathogenic; “P” for pathogenic) (see picture 3).
Picture 3: Example of variant classification in the new report from CeGaT
In the example in the table (picture 3), the variant (ZBTB20, c.1832G>A; p.Cys611Tyr) is scored with 11 points after evaluation of the ACMG criteria and is therefore classified as pathogenic. Accordingly, the dark red field “P” (“Pathogenic”) is shown enlarged and makes the classification detectable at a glance.
Regular re-evaluation of VUS
We ensure that variants of unclear significance (VUS) are always classified based on currently available evidence. If the classification of a variant changes due to new evidence and in relevance to your patient, we will proactively inform you as part of our VUS re-evaluation. We will provide you with an updated report for your patients, upon your confirmation.”
To ensure that our high quality standards are met, we perform every step from sample receipt to report generation in-house. We are accredited as a human genetic diagnostic laboratory according to DIN EN ISO 15189 and the American standards (CAP/CLIA).
Learn more about our in-house diagnostics here and benefit from our comprehensive diagnostic services together with your patients.
We look forward to your questions and will be happy to advise you. Contact us at diagnostic-support@cegat.com.
References
- Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in medicine : official journal of the American College of Medical Genetics 17, 405–424; 10.1038/gim.2015.30 (2015).
- Ellard, S. et al. ACGS Best Practice Guidelines for Variant Classification in Rare Disease 2020. Association for Clinical Genomic Science 32 (2020).
- Tavtigian, S. V., Harrison, S. M., Boucher, K. M. & Biesecker, L. G. Fitting a naturally scaled point system to the ACMG/AMP variant classification guidelines. Human mutation 41, 1734–1737; 10.1002/humu.24088 (2020).