Liquid biopsy analysis detects cell-free DNA (cfDNA) released into the bloodstream by necrotic and apoptotic cells and thus offers an optimal alternative when tumor tissue is unavailable or for disease monitoring. However, only a fraction of circulating DNA originates from the tumor itself. Therefore, highly sensitive methods are required to detect these minimal cfDNA concentrations.
We at CeGaT established our CancerDetect® panel using duplex UMI-based technology, which detects sequence variants in actionable, most prevalent hotspots. In addition, the sampling is non-invasive and easily repeatable, making CancerDetect® an excellent approach for monitoring analysis. By very sensitive detection of tumor-specific biomarkers, the analysis of cfDNA can be used as a surrogate marker for treatment response during medical follow-up.
For many liquid biopsy-based somatic analyses, it is helpful to look at changes in the results over time and interpret them in the clinical context. With the new monitoring report layout, the data from serial CancerDetect® analyses are summarized in one report and can be interpreted at a glance. The results of each additional sample are integrated into existing reports. An updated progression graph and corresponding tables clearly show a trend of the monitored changes based on the variant frequency. Conclusions can be drawn about the course of the disease, and therapy success and the occurrence of resistance mutations can be recognized in time.
Are you insured in Germany? Our colleagues at the Zentrum für Humangenetik Tübingen will gladly support you!
Ideally Suited for Monitoring and Follow-up
Our Promise to You
Service Details
- Liquid biopsy enables the detection of variants with potential therapeutic relevance in patients where the tumor is inaccessible, allowing to gain information about the tumor and to address treatment (see sample report “single/first sample”). — Learn more
- Contains a list of all eligible drugs, with EMA and/or FDA approval, for which corresponding biomarkers could be detected in the tumor (see sample report “single/first sample”). — Learn more
- Highly sensitive and accurate detection of actionable, most prevalent driver mutations in thirty-one genes and gene fusions in 6 genes with very low allele frequencies by using duplex UMI-based technology (NAF ≥ 0.25%)
- High coverage: 50,000–100,000x raw coverage
- Simple, non-invasive, and repeatable sampling provides the best conditions for longitudinal follow-up testing and disease monitoring.
- By very sensitive detection of tumor-specific biomarkers, the analysis of cfDNA can be used to track tumor dynamics in real-time and intervene or adjust treatment if necessary (e. g., at acquired drug resistance).
- In the case of serial sampling, the results of previous analyses are automatically taken into account, and the progression is depicted clearly in a figure. The focus here is on possible changes in the variant frequencies of important driver mutations over time (see sample report “Monitoring”).
- The monitoring system has a flexible structure and considers the frequency change of each somatic variant separately. Therefore, it is possible to integrate newly occurring changes into the progression report at any time.
We will report all relevant findings in a medical report. This includes a list of all identified clinically relevant variants. The report contains a list of all identified clinically relevant variants and the therapeutic approaches derived from them for each first sample of a series as well as for individual analyses with only one sample collection time examined.
Every single medical report is prepared and discussed by an interdisciplinary team of scientists and physicians to guarantee the highest quality.
Sample Report „Single/First Sample“
Sample Report „Monitoring“
Our Standard Sample Requirements
Liquid Biopsy
Liquid Biopsy samples are specimens that can only be withdrawn using special collection tubes that stabilize the cell-free DNA. If you are planning a diagnostic examination based on cfDNA, please use such collection tubes. We gladly provide such special collection tubes. Please contact us in time at to order the tubes.
- 3x 10 ml cfDNA tubes for liquid biopsy (for blood as the recommended sample type)
If cerebrospinal fluid or fluid from cysts is to be used as starting material, please reduce the amount taken accordingly if necessary)
Type of Primary Sample for cfDNA Isolation
- Blood
- Ascites
- Liquor
- Pancreatic cyst fluid
Contact us if you would like to analyze other sample types.
Notes on Sample Shipment
Here you can find more information on how to ship your sample safely.
This Is What Makes Our CancerDetect® Service Special
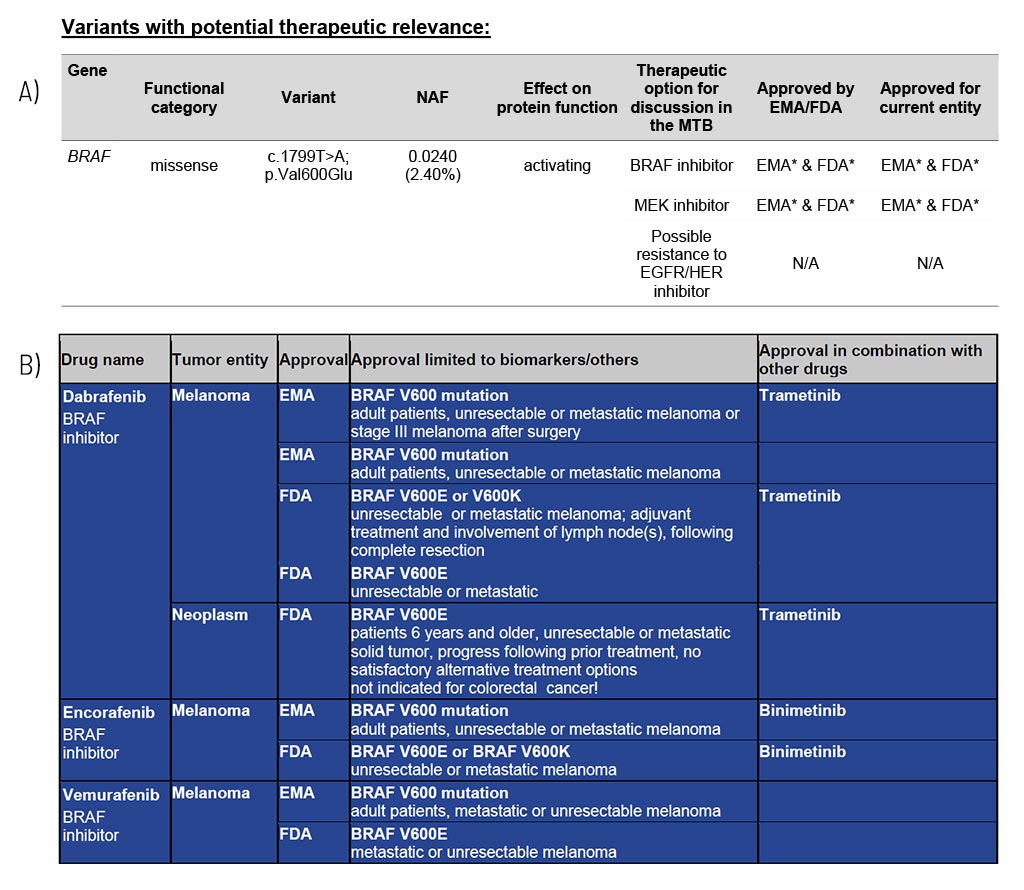
Variants with Potential Therapeutic Relevance
Guidance on potentially effective drugs
For each gene, the somatic change is depicted in detail, and the resulting therapeutic options are stated, including the EMA/FDA approval (A). These options are the basis for discussion in a molecular tumor board (MTB).
At the end of the medical report, in the appendix/supplement, we provide an extensive list of possible therapeutic strategies for each identified somatic changes (B). This list includes drug classes and names as well as their approval (FDA/EMA) and limiting conditions. The overview of therapeutic options is shown in full in every first report of a serial follow-up diagnosis.
Sample Report: Exemplary for the BRAF variant detected and the resulting therapeutic options in a patient with melanoma. Top panel (A): An excerpt from Table 1 of the findings, listing variants with therapeutic relevance. Lower part (B): An excerpt of the drug listing. In addition to the drugs shown, other drugs are also described.
CancerDetect® Monitoring Applications
For serial sampling, progress reports are automatically generated from the second sample onwards. Additional sample reports can integrate any number of previous analyses from liquid biopsy samples. Thanks to its clear illustrations and tables, the progress report allows changes in the occurrence of monitored tumor mutations to be identified at a glance. Indications of recurrence can thus be detected at an early stage, patients with stable disease can be monitored regularly, and the occurrence of resistance mutations during treatment can be immediately considered in the treatment plan.
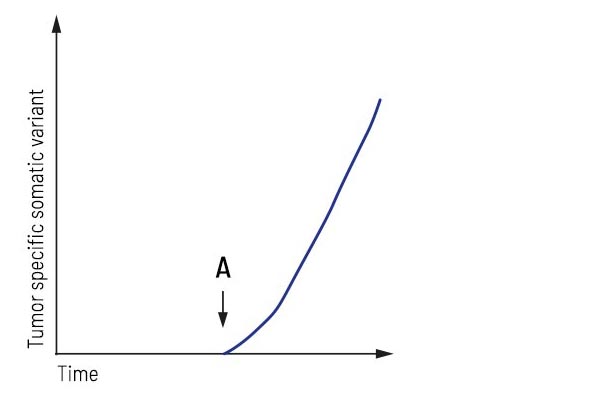
Application “Relapse detection”:
After surgery/treatment, the patient was considered tumor-free. Regular liquid biopsy testing revealed tumor progression, showing that an increase in a tumor-specific variant accompanied the recurrence of the tumor. This biomarker’s highly sensitive detection may detect the tumor’s relapse earlier than conventional imaging techniques.
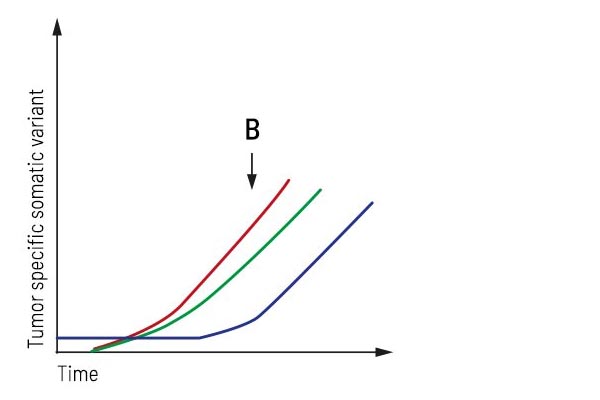
Application “Monitoring during treatment”:
After treatment, the patient underwent strong regression resulting in stable disease. Longitudinal monitoring provides an up-to-date molecular profile of the tumor and detects emerging treatment resistance in time. Here, two additional subclones occur besides the primary tumor mutation, forcing the treatment to be adjusted appropriately.
CancerDetect® Sample Case
Patient and indication:
- 42 years old, female, metastatic non-small cell lung cancer (NSCLC) with an EGFR L858R mutation in the primarius
- relapse after initial response to afatinib treatment
- recurrent tumor inoperable, no tumor biopsy possible
Primary finding:
The result of an analysis of cell-free DNA using a standard lung cancer panel remained negative.
CancerDetect® finding:
Our CancerDetect® analysis revealed a 2% tumor content in the liquid biopsy and detected the known EGFR L858R mutation, as well as an additional EGFR T790M resistance mutation. The EGFR T790M mutation represents one of the most common resistance mechanisms to tyrosine kinase inhibitors (TKIs) and typically occurs in NSCLC patients after first-line TKI treatment. In this patient, treatment was adjusted with the third-generation EGFR inhibitor osimertinib.
Gene Directory
All relevant variants in a named exon are analyzed. Exon numbers refer to coding exons (CDS) of the respective gene. The diagnostic is not limited to the listed example hotspot mutations. Exons not named and all variants within are not part of the analysis.
Gene | NM_Nr. | Enriched region (incl. example hotspot (HS)-variants) |
AKT1 | NM_005163.2 | Exon 2 (incl. HS E17) |
ALK | NM_004304.5 | Exons 22-25 (incl. HS F1174 , G1202, F1245, |
AR | NM_000044.6 | Exons 4, 5, 8 |
BRAF | NM_004333.6 | Exons 11, 15 (incl. HS V600) |
CDKN2A | NM_000077.5 | Entire coding region |
CTNNB1 | NM_001904.4 | Exons 2, 6, 7 (incl. HS S37, S45, K335, N387) |
EGFR | NM_005228.5 | Exons 2, 3, 6, 7, 15, 18-21 |
ERBB2 | NM_004448.4 | Exons 8, 17, 19-21 (incl. HS S310, R678, V842) |
ERBB3 | NM_001982.4 | Exons 3, 7-9, 23 (incl. HS V104, E928) |
ESR1 | NM_000125.4 | Exons 4, 5, 7, 8 (incl. HS K303, Y537, D538, E380Q, |
FGFR1 | NM_023110.3 | Exons 11-13 (incl. HS N577, K687) |
FGFR2 | NM_000141.5 | Exons 6, 8, 11-13 (incl. HS S252, N549) |
FGFR3 | NM_000142.5 | Exons 6, 8, 13 (incl. HS R248, S249, Y375) |
GNA11 | NM_002067.5 | Exons 4, 5 (incl. HS R183, Q209) |
GNAQ | NM_002072.5 | Exons 2, 4, 5 (incl. HS T96, R183, Q209) |
Gene | NM_Nr. | Enriched region (incl. example hotspot (HS)-variants) |
GNAS | NM_000516.7 | Exon 8 (incl. HS 201) |
H3-3A | NM_002107.7 | Exon 1 (incl. HS K27, G34) |
HRAS | NM_005343.4 | Exons 1-3 (incl. HS G12, Q61) |
IDH1 | NM_005896.4 | Exon 2 (incl. HS R132) |
IDH2 | NM_002168.4 | Exon 4 (incl. HS R140, R172) |
JAK2 | NM_004972.4 | Exon 12 (incl. HS V617) |
KIT | NM_000222.3 | Exons 9, 11, 13, 14, 17, |
KRAS | NM_004985.5 | Exons 1-3 (incl. HS G12, G13, Q61) |
MET | NM_001127500.3 | Exons 13, 15, 18 (incl. HS L982_D1028del, T1010, |
NRAS | NM_002524.5 | Exons 1-3 (incl. HS G12, Q61) |
PDGFRA | NM_006206.6 | Exons 11, 13, 17 (incl. HS D842) |
PIK3CA | NM_006218.4 | Exons 1, 4, 7, 9, 13, 20 |
PTEN | NM_000314.8 | Entire coding region |
RET | NM_020975.6 | Exons 10, 11, 13-16 (incl. HS C634) |
TERT | NM_198253.3 | Promotor HS c.-124 (C228), c.-146 (C250) |
TP53 | NM_000546.6 | Entire coding region |
Dna-Based Detection of Selected Structural Variations in These Genes
ALK, RET, ROS1, FGFR2, FGFR3, NTRK1
Further Information
Webinar: Discover the Power of Modern Tumor Diagnostics
Liquid biopsy in genetic tumor diagnostics – CancerDetect®
Contact Us
Do you have a question, or are you interested in our service?
Diagnostic Support
We will assist you in selecting the diagnostic strategy – for each patient.