Our Tumor Diagnostics Portfolio
What Is Genetic Tumor Diagnostics?
Cancer arises from changes in genes that deregulate cell growth and cause cells to grow out of control. Genetic tumor diagnostics hence is a powerful tool as it detects predispositions for cancer, identifies existing diseases and gives insights into the disease mechanism for optimal treatment decisions.
A valid diagnosis improves the patient’s quality of life drastically. It provides clarity and guidelines for action to lower the risk of developing cancer or to detect the disease early.
Based on the information gained, physicians who treat patients with cancer can determine the most effective therapy to increase the chances of recovery.
Genetic diagnostics should be integrated early into the diagnostic plan – because the right diagnosis is the basis for optimal management of cancer.
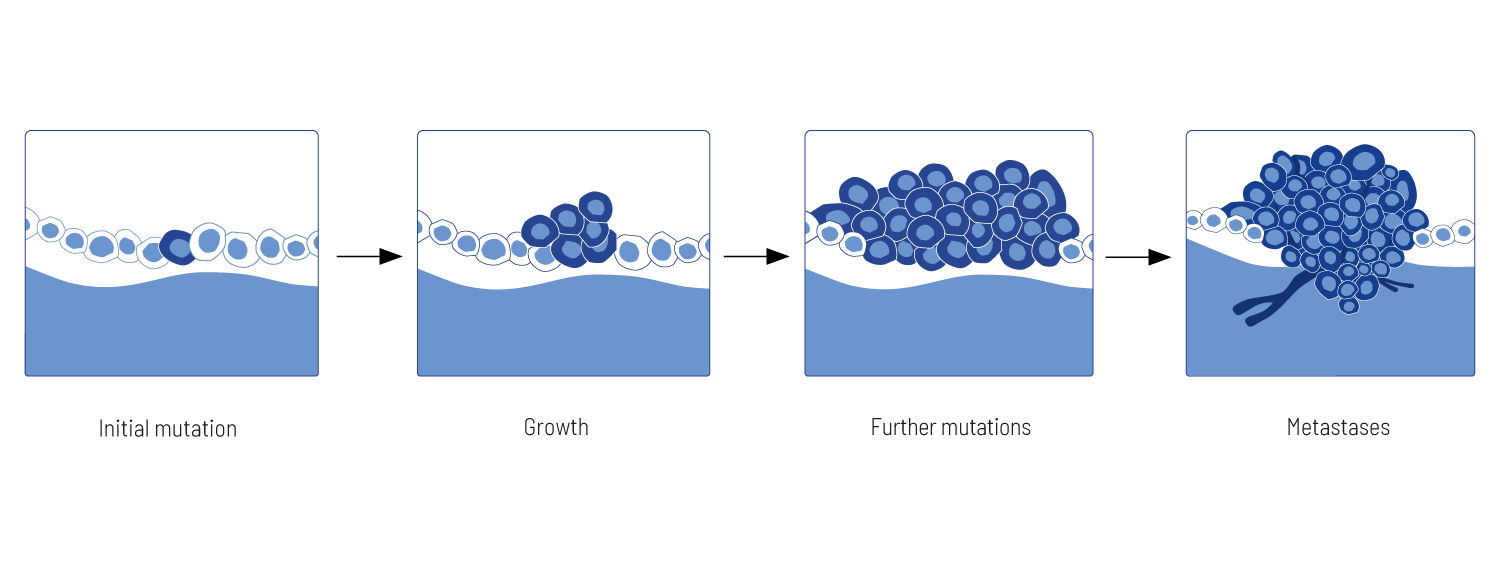
Tumor Development
Tumors develop from cells as a consequence of mutations in genes. Mutations usually occur randomly and are typically corrected by the cell’s DNA repair systems. However, exposure to carcinogens, such as tobacco smoke or UV radiation increases the number of mutations. A not correctly repaired mutation can result in altered protein function, leading to dysfunction of cellular processes and possibly the beginning of cancer. Spontaneously obtained mutations are called somatic mutations. The genetic alterations initially drive the loss of growth control, the proliferation of cell clones with reduced mortality rate, and finally the distribution of tumor cells to distant organs (metastases). The somatic mutations acquired by tumors in the course of the disease are individual and differ not only between different cancer entities but also from patient to patient – every tumor is unique. Thus, cancer is a multifactorial and heterogeneous genetic disease.
NGS-Guided Oncogenetics — A New Era of Cancer Management
The individual set of tumor-specific mutations helps the tumor to survive and develop resistance against therapeutic agents. To choose a promising treatment strategy, a deep and accurate look into the molecular underpinnings of individual tumors is required. The use of next-generation sequencing (NGS) has initiated a new era in cancer therapy and is a driving force to change the future in personalized precision medicine. Through NGS analysis of genetic mutations, we can tailor the oncological treatments to each patient’s features and each cancer genomic alterations to maximize the curative effect, minimize damage to healthy tissues, and optimize resources.1, 2, 3
Genetic Tumor Diagnostics Is the Decision-making Basis for Choosing the Best Possible Analysis Strategy
Genetic analysis of the tumor is essential for choosing the optimal treatment. The table below describes the variety of genetic, molecular profiling tests offered. The choice of test depends on the samples available and, in particular, on the objective of the desired diagnosis.
Full molecular analysis | Identification of neoantigens | Focused analysis | Focused analysis / Monitoring | |
Sample Type | Solid tumor tissue / | Solid tumor tissue | Solid tumor tissue | Liquid biopsy |
Scope | > 700 genes and fusions | Whole exome and transcriptome sequencing | Disease specific gene sets covering up to 54 genes and selected structural variants in 9 genes | Hotspot regions in 31 genes and fusions in 6 genes (TP53 full coding) |
Normal tissue comparison | ✓ | ✓ | ✗ | ✗ |
Detailed drug options list | ✓ | ✓ | ✓ | ✓ |
Detection threshold (NAF) | 5% | 5% | 5% | 0.25% |
Minimal tumor content | 20% | 20% | 20% | 1% |
SNV & INDELS | ✓ | ✓ | ✓ | ✓ |
CNV | ✓ | ✓ | ✗ | ✗ |
TMB | ✓ | ✓ | ✗ | ✗ |
MSI | ✓ | ✓ | ✓ (extra order) | ✗ |
HRD | ✓ | ✓ | ✗ | ✗ |
Fusion gene analysis / Structural variants (DNA-based) | ✓ | ✓ | ✓ | ✗ |
Additional option: RNA-based fusion transcript analysis (CancerFusionRx®) | ✓ (only with FFPE) | ✓ | ✓ | ✗ |
HPV/EBV/MCV/CMV infection | ✓ | ✓ | ✗ | ✗ |
HLA typing | ✓ | ✓ | ✗ | ✗ |
Neoantigen | ✗ | ✓ | ✗ | ✗ |
Pharmacogenetics variants (selection) | ✓ | ✓ | ✗ | ✗ |
CHIP Detection | ✓ | ✓ | ✗ | ✗ |
Representation of biological pathways and coverage profile (copy number variations) | ✓ | ✓ | ✗ | ✗ |
The Power of Liquid Biopsy Testing
Access to Tumor by Blood Analysis
Liquid biopsy analysis represents an optimal alternative testing procedure in cases where tumor tissue is unavailable, e. g., irresectable tumors or poor patient conditions. The main target of liquid biopsy analysis is cell-free DNA (cfDNA) which is increasingly released into the bloodstream by necrotic and apoptotic cells in patients with cancer and other types of diseases. Circulating cfDNA is released from both normal and tumor cells. The fraction of tumor-derived cfDNA (circulating tumor DNA/ctDNA) depends on tumor entity, tumor stage, tumor burden, and other factors and therefore is not the same in every patient. Since only a fraction of circulating cfDNA is derived from the tumor, highly sensitive methods are required to detect minimal ctDNA concentrations.
CeGaT has established and validated extensive liquid biopsy panels assessing cell-free DNA. CancerPrecision® uses liquid biopsy testing to provide a complete genetic profile of the patient’s tumor, supporting the choice of the best possible therapy. A tumor content of at least 20% is required for this analysis. Since the content of ctDNA can be less than 20%, CeGaT also offers CancerDetect®, a duplex UMI-based panel. CancerDetect® identifies sequence variants in actionable, most prevalent driver mutations with an allele frequency above 0.25%. This highly sensitive detection of tumor specific biomarkers represents an excellent tool for monitoring treatment response and minimal residual disease. In addition, the method’s high sensitivity makes failure much less likely, even with a low tumor fraction in the sample.
*Unique molecular identifier
Advantages of liquid biopsy
- Non-invasive
- Rapid and precise diagnosis
- Real-time monitoring of therapies and early detection of relapse or progression
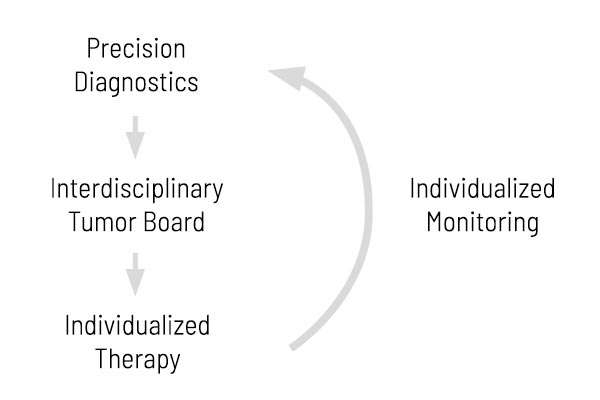
Integration of Genetic Tumor Diagnostics In Patient Management
Our Somatic Tumor Analyses Accompany You and Your Patients Optimally — From Diagnosis to Monitoring
Time is most precious in cancer patient treatment. The earlier a cancer is detected, and the earlier an effective treatment is used, the better the chances for the patient. Understanding the molecular detail of the tumor as early as possible offers the chance to use an effective treatment at an early stage. In our opinion, the best strategy is to start with a detailed diagnostic approach that includes our CancerPrecision® analysis to address the genetic changes of the tumor.
Then, the results should be discussed in a local interdisciplinary MTB. This board decides on the most promising treatment strategy based on all available information on the tumor and the patient. Finally, this individualized therapy should be applied, and the patient should be monitored for success during treatment. Depending on the individual case, this monitoring should include genetic markers, like our CancerDetect®, to address potential resistance mechanisms or detect recurrences early.
References
1 Walter, C. et al. Sequencing for an interdisciplinary molecular tumor board in patients with advanced breast cancer: experiences from a case series. Oncotarget 11, 3279–3285; 10.18632/oncotarget.27704 (2020).
2 Morganti, S. et al. Role of Next-Generation Sequencing Technologies in Personalized Medicine. In P5 eHealth: An Agenda for the Health Technologies of the Future, edited by G. Pravettoni & S. Triberti (Springer International Publishing, Cham, 2020), 125–154; 10.1007/978-3-030-27994-3_8 (2020).
3 Wu, T.-M. et al. Power and Promise of Next-Generation Sequencing in Liquid Biopsies and Cancer Control. Cancer control : journal of the Moffitt Cancer Center 27, 1073274820934805; 10.1177/1073274820934805 (2020).
Our Accreditations
Binding standards guarantee the quality of our work: Our laboratory services are accredited according to CAP/CLIA and DIN EN ISO 15189. You can find further accreditations and certifications here.
You Are also Welcome to Take a Look at the Following Areas
Contact Us
Do you have a question, or are you interested in our service?
Diagnostic Support
We will assist you in selecting the diagnostic strategy – for each patient.