Minimal residual disease testing (MRD) refers to the small number of circulating tumor DNA (ctDNA) molecules in the bloodstream during or after treatment originating from the smallest amounts of residual cancer cells in the body. MRD monitoring is done by checking for circulating tumor DNA, ctDNA, released by tumor cells into the bloodstream. This type of monitoring is becoming a new alternative to dynamically check for treatment effectiveness and predict possible recurrence in cancer patients. Disease monitoring using liquid biopsy as a sample type allows for a repeatable and non-invasive sample collection process for the patients and the detection of a possible relapse at an earlier stage, for example, compared to imaging.
With CancerMRD, we perform personalized and tissue-informed monitoring of MRD for solid tumors via whole genome sequencing (WGS), which ensures a high sensitivity with the wide range detection of tumor-specific variants across the entire genome.
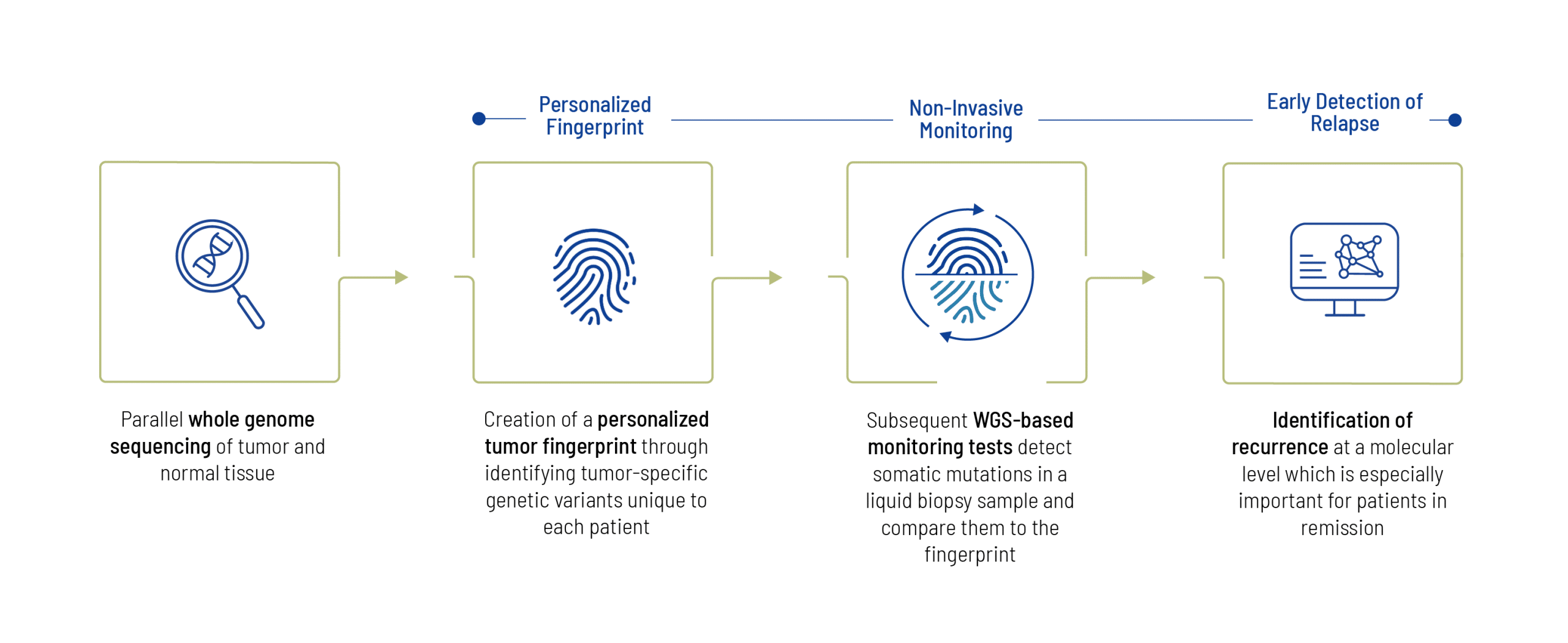
CancerMRD involves two main components: initial fingerprint of the tumor, where the solid tumor tissue and EDTA blood are sequenced using WGS, and tumor-specific variants are detected, excluding germline variants. The next step is subsequent monitoring of the tumor-specific variants using liquid biopsy. The monitoring reports include information on detected tumor percentages and a progression graph, providing a visual representation of ctDNA detection throughout performed tests.
Tissue-informed MRD Monitoring for Solid Tumors
Our Promise to You
Service Details
- Tissue-informed approach detects tumor-specific mutations via WGS, analyzing both tumor and normal tissue. For this initial fingerprinting, a solid tumor sample and a blood sample (EDTA blood) is required.
- Subsequent MRD monitoring only requires liquid biopsy samples and uses existing fingerprint.
- Simple, non-invasive, and repeatable sampling provides the best conditions for longitudinal follow up testing and monitoring.
- With serial sampling, previous monitoring results are incorporated into the findings and depicted in a visual graph to illustrate progression.
- Using CancerMRD, a tumor content of 0,1% and lower can be detected in a liquid biopsy sample.
- Long-term monitoring tracks disease progression, which can be used for treatment-relevant decisions (such as adjuvant treatment or de-escalating treatment given continuously negative results) and early detection of a potential relapse for patients in remission.
In order to start monitoring, the initial fingerprint of the tumor is necessary, which requires the availability of a solid tumor sample. After the tumor-specific variants are detected by tumor vs normal tissue comparison, further tests require only blood samples (liquid biopsy). Sample reports of each step are included below.
This test is designed for MRD monitoring of solid tumor entities that shed ctDNA into the blood stream. It is not indicated for entities that have limited ctDNA shedding.
Sample Report “Fingerprint”
Sample Report “Monitoring”
CancerMRD Process Overview
Our Standard Sample Requirements
For the Initial Fingerprint of the Tumor
Normal Tissue
- 1–2 ml EDTA blood (recommended sample type) or
- Genomic DNA (1–2 μg)
Tumor Tissue
Tumor content at least 20%
- FFPE tumor block (min. tissue size 5 x 5 x 5 mm) (recommended sample type) or
- FFPE tumor tissue slides (min. 10 slices 4-10 μm, tissue size 5 x 5 mm) or
- Genomic DNA (> 200 ng)
For MRD Monitoring
For each subsequent testing time point, we require a new sample, namely liquid biopsy
- 3x 10 ml cfDNA tubes for liquid biopsy (for blood as the recommended sample type)
Liquid Biopsy samples are specimens that can only be withdrawn using special collection tubes that stabilize the cell-free DNA, so please make sure to use such specialized collection tubes. We can also provide these upon request, the relevant contact form can be found here.
Further Sample Materials
Other sample options are possible on request. Please note: in case of insufficient sample quality or tumor content, the analysis might fail.
If you have more than one option of tumor samples, please get in touch with us (tumor@cegat.com), and we would be happy to assist you in choosing the optimal specimen for your patient.
Here you can find more information on how to ship your sample safely.
This Is What Makes Our CancerMRD Service Special
Tissue-informed Personalized Tumor Fingerprint
There are a few different approaches in MRD monitoring. Some use a so-called “tissue-naïve” approach, where the MRD monitoring is focused on common tumor alterations that are likely to be present in a given tumor entity rather than a personalized approach. Here at CeGaT, we are proceeding with a tissue-informed approach, where we initially sequence both tumor and normal tissue to detect tumor-specific mutations, providing a personalized tumor fingerprint.
Analysis through whole genome sequencing (WGS) allows for MRD detection on a much broader scale, identifying many more tumor-specific alterations than panel sequencing. These alterations have no relevance for treatment or prognosis, but including them in the fingerprint results in a much larger fingerprint size, and thus to higher sensitivity of MRD detection. In comparison to tissue-naïve approaches, the tissue-informed fingerprints contain patient-specific variations that are too rare to be included in generic fingerprints.
CancerMRD Monitoring Applications
There are many ongoing studies to define uses of MRD in clinical setting for tumor patients. We consider the following points as the key applications of MRD monitoring.
Treatment Decision and Response Monitoring
MRD monitoring offers a dynamic assessment of tumor behavior at a molecular level, enabling real time monitoring of tumor burden and how the tumor is responding to ongoing treatment. This can provide insights into how much residual tumor DNA is present after surgery or treatment, guiding decisions on whether additional steps – such as changes in treatment regime, adjuvant treatment or de-escalation – should be considered.
For example, if MRD is detected after primary treatment, it may suggest incomplete response, leading clinicians to escalate treatment or opt for consolidation therapy to target remaining cancer cells. Conversely, the absence of MRD after treatment could support decisions to reduce or stop further treatment, potentially avoiding overtreatment and reducing side effects.
MRD detection can also guide the use of adjuvant therapies, such as chemotherapy or targeted therapies. MRD-positive patients may benefit from more intensive adjuvant treatment to minimize the risk of relapse, while MRD-negative patients might avoid unnecessary treatments.
Estimation of Tumor Progression
MRD monitoring can detect the presence of residual tumor cells that may not be visible on imaging scans, even after a seemingly successful primary treatment. The presence of MRD may indicate the persistence of subclinical disease, suggesting that tumor progression is imminent.
MRD detection allows for the identification of patients at higher risk of relapse or progression. If MRD is detected in a patient after treatment, they may be more likely to experience recurrence. This information can help tailor continuous monitoring strategies to catch tumor progression in a timely manner.
Early Detection of a Possible Relapse
Since MRD can detect circulating tumor DNA, relapse at a molecular level can be detected before it becomes clinically apparent. This is especially important in cancers where relapse can occur even after a prolonged period of remission.
Relapses can also be detected by imaging techniques (e.g., CT scans, MRIs) but these only detect growths of a certain size. Newly emerging symptoms can also indicate tumor relapse, usually appearing only with much larger growth. MRD, on the other hand, can identify very small amounts of residual tumor DNA in the bloodstream, enabling the detection of relapse at an earlier stage. This allows for timely interventions and adjustments in monitoring frequency, ultimately improving overall patient outcomes in case of relapse.
Contact Us
Do you have a question, or are you interested in our service?
Diagnostic Support
We will assist you in selecting the diagnostic strategy – for each patient.




